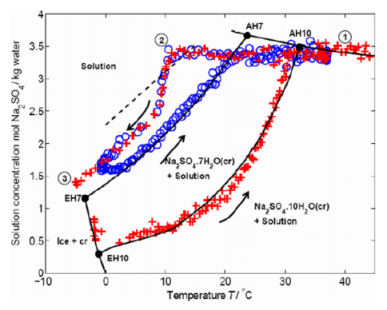
Figure 1: The measured sodium sulfate concentration as a
function of the temperature.

Figure 1: The measured sodium sulfate concentration as a
function of the temperature.
As can be seen up on cooling the sample starts to supersaturate
first with respect to mirabilite and later with respect to
heptahydrate. As coon as the concentration reaches the spontaneous
crystallization line, heptahydrate is formed and the concentration
drops fast. In one experiment after reaching 0 oC
the temperature is increased again and the
concentration is following the equilibrium heptahydrate
line. Whereas in the other experiment the sample was
further cooled and upon reaching -4 oC a
transformation from heptahydrate into mirabilite is observed
and upon heating up the equilibrium line of mirabilite is
followed.
These experiments were also reproduced in an XRD experiment. These experiments were conducted on the beamline 16.4 at the UK Synchrotron Radiation Source, Daresbury. The resulting XRD diffraction patterns are given in figure 2.
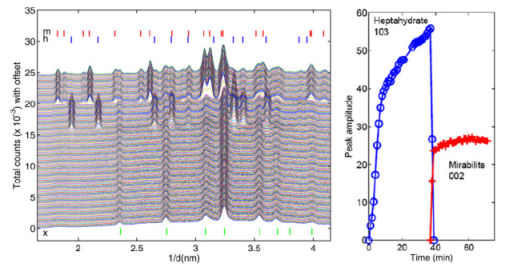
Figure 2. X-ray diffraction patterns as obtained during
cooling.
As can be seen there again a very fast formation of heptahydrate is observed, whereas also the transformation into mirabilite can clearly be seen. Hence these independent measurements clearly confirm the formation of heptahydrate in a porous material.
Andrea Hamilton, Christopher Hall and Leo Pel, Sodium sulfate
heptahydrate: direct observation of the crystallization in a
porous material, J. Phys. D:
Appl. Phys. 41
212002 (2008).