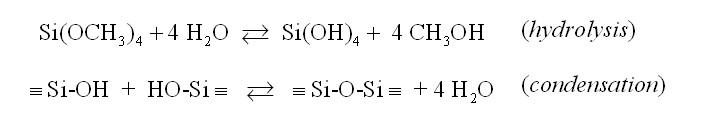
Reactive
transport and gel formation in two-phase systems and porous media
Introduction
Reactive transport phenomena occur in a wide variety of
scientific and engineering fields. Coupled mass transfer and chemical
reactions are found in chemical reactors, biological cells, soils, etc.
An interesting example of reactive flow is found in the application of
gel systems in porous rocks, in order to modify the fluid flow
properties in the rock, with respect to oil or water. High water-cut
during oil and gas production is a world-wide problem, especially in
maturing oil fields, and leads to a decline in hydrocarbon production
and to water disposal problems. Gel treatments can be applied in the
near-well bore region to reduce or block the flow of water into the
well. Gels have also been considered for their potential use as a
barrier to contaminant transport in groundwater.
A novel type of gelant was introduced by
Thompson and Fogler,that can be mixed with oil,
and reacts upon contact with water to form a gel in the water phase.
This gelant, TMOS or Si(OCH3)4, reacts with
water as according to the sol-gel principle:
With respect to the application of the gelant in two-phase systems (in bulk or in porous media) the gelant TMOS is initially mixed with oil or a hydrocarbon. Near the oil-water interface the gelant will transfer to the water phase and react with water to form a gel. This process is shown in Figure 1.

NMR bulk
experiments
The
coupled mass transfer and gel reactions were studied in bulk systems
containing both oil and water. Two series of experiments were
performed. The first one was done using n-hexadecane (for the oil
phase) and normal water (for the water phase). The reactive transport
was monitored using a 4.7 Tesla NMR scanner. The second series was done
using a mineral oil and heavy water (D2O) with or without a
buffer. In this series the effect of pH on the reactive transport
mechanisms was analyzed. These experiments were done using a 0.95 Tesla
NMR scanner equipped with a binuclear rf
insert. The hardware was custom made to allow for fast toggling between
both components.
2D images were acquired, using the 4.7 Tesla scanner,
to obtain a qualitative view on the process and to obtain a measure for
the rate of mass transfer that is directly indicated by the shift of
the oil-water interface. The images are T1-weighted to yield an
adequate contrast between water and oil/TMOS (see Figure 2). From these
2D images the interfacial tension between both phases can be determined
by employing a detailed image analysis and optimization procedure. Our
analysis showed that the interfacial tension changes during the
reactive transport. This is relevant with respect to the two-phase flow
processes in porous media.
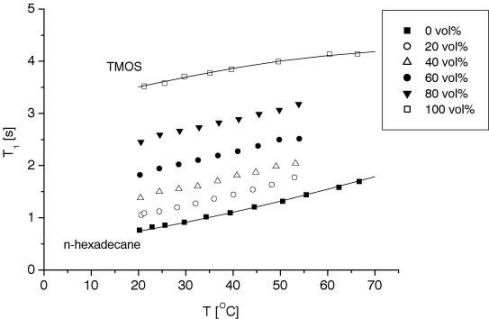
Fig. 2. NMR images of the
two-phase bulk system (vertical slice of a cylindrical sample),
acquired at the
beginning of the
experiment (left frame), and after 15 hours (right frame).
The upper phase represents the oil/TMOS phase (initial f = 40 vol%),
and the lower phase represents the
(gelled) water phase.
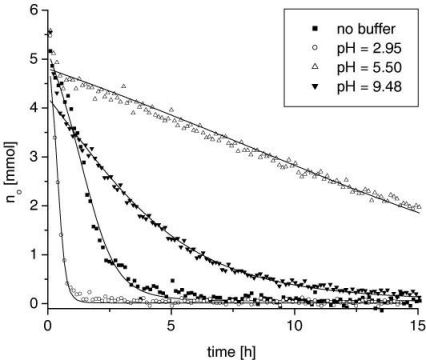
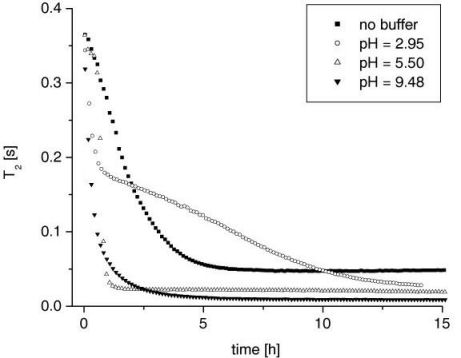
Additionally, in the bulk systems the concentration of the gelant can be monitored by measuring the T1 of the mixture. For this a calibration of the relaxation time T1 for different concentrations was obtained (see Figure 3). In the second series (with the D2O buffers) it was observed that the change in concentration, i.e. the mass transfer, is driven by the hydrolysis reaction, the rate of which is a strong function of pH and temperature. Figure 4 shows the concentration plots for different pH systems.

NMR
experiments on
reactive transport in porous materials

Effect of gel placement on the permeability
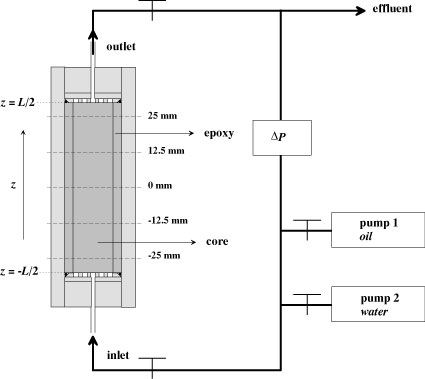
Parallel to the NMR
experiments with the sandstone cores, as described above, the effect of
the gel
on the relative permeabilities was determined. This was done by
monitoring the
differential pressure (see Figure 6) over the cores, while injection
oil or
water. The results showed that the relative permeability to oil was
reduced up
to a factor of 3.2. The relative permeability to water was reduced by a
factor
between 1.9 and 27. For each experiment, the relative permeability to
water was
reduced more than that to oil. No clear dependence of the reduction on
the
parameters (pH, temperature) was observed within the range considered.
The disproportionate
permeability reduction is advantageous for the water shut-off treatments.
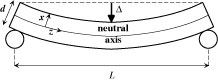
A series of beam-bending
experiments was performed to study the effect of in situ
formed gel (under single phase) conditions on the overall permeability
of the
sandstone. Beam bending (see Figure 8) is an excellent method to
measure the permeability
in low-permeable media, such as gels, concrete etc.

Castelijns,
H.J., L. Pel, H.P. Huinink, P.L.J. Zitha, Mass transfer and gelation in
sandstone cores of a novel water shut-off chemical, conference paper
SPE 99684
presented at the 2006 SPE/DOE Symposium on Improved Oil Recovery, held
in
Tulsa, Oklahoma, USA, 22-26 April 2006.
Castelijns,
H.J., H.P. Huinink, P.L.J. Zitha, Characterization of interfacial
effects
during reactive transport with MRI methods, Colloids & Surfaces
A, in
press (2007).