SALT
TRANSPORT IN PLASTER/SUBSTRATE LAYERS
We have investigated how transport and accumulation of salt in a
plaster
depends on the underlying masonry material. Therefor the same
plaster
is
applied on two substrates of which the pores are either an order
of
magnitude
larger or smaller than those of the plaster, i.e., a plaster layer
(lime
: cement : sand = 4 : 1 : 10) on a Bentheimer sandstone or on
calcium
silicate
brick. The poresizes as measured for the various layers using
mercury
porosimetry
is given in table 1.
| Material |
nano-pores
|
micro-pores
|
volume ratio
|
| Bentheimer
sandstone |
¾
|
(40 ± 20) m
m
|
¾
|
| Calcium-silicate
brick |
(20 ± 10) nm
|
(20 ± 10) m
m
|
1:1
|
| Plaster |
¾
|
(0.7 ± 0.4) m
m
|
¾
|
Table 1. Pore sizes measured by mercury-intrusion
porosimetry.
Initially the samples were saturated with a salt-solution of
different
concentrations. Due to the differences in pore structure between
the
sandstone
and the calcium silicate brick totally different drying and
crystallization
behavior is observed. In figure 1 the measured moisture
distribution
for plaster/Bentheimer sandstone as a function of time is given.
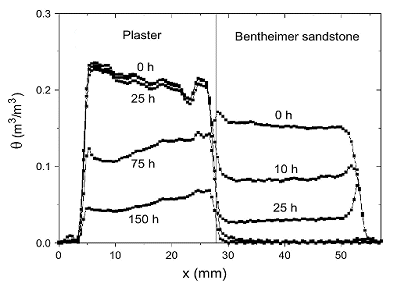
Figure 1: Moisture content in the plaster/Bentheimer
sandstone
system
during drying. The
sample was initially saturated with a NaCl solution (c = 4
mol/l
). Dry air is blown
over the top of the sample (x = 4 mm) with a flow of 0.7 l/
min
.
In a Bentheimer sandstone the pores are much smaller than the
substrate
and hence it is drying first. In figure 2 the the Na content as a
function
of time is given.
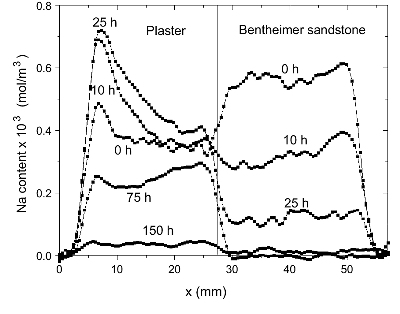
Figure 2. The Na content a function of time for
plaster/Bentheimer
sandstone system during drying.
As can be seen in this case almost all salt is transported from
the
sandstone into the plaster, i.e, all salt is removed from the
sandstone.
In figure 3 the measured moisture distribution for plaster/calcium
silicate brick as a function of time is given. Due to the fact
that in
this case the brick contains a large number of pores smaller
than
the plaster, first the plaster layer dries out.
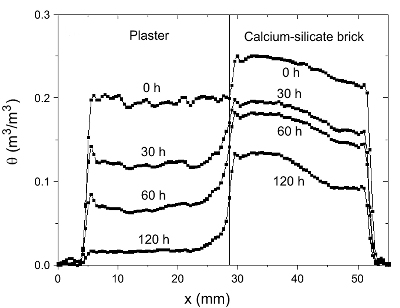
Figure 3: Moisture content in the plaster/calcium-silicate
brick
system during drying. The
sample was initially saturated with a NaCl solution (c = 4
mol/l
). Dry air is blown
over the top of the sample (x = 4 mm) with a flow of 0.7 l/
min
.
In this case a significant amount of salt will stay in the brick
and
crystallize there. Hence the efficiency for salt removal is very
low
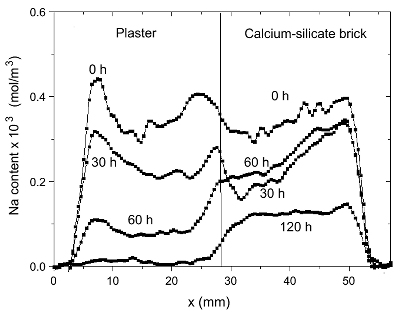
Figure 4. The Na content a function of time for
plaster/calcium-silicate
brick
system during drying.
From these we can conclude that the performance of the plaster is
not
solely determined by the properties of the plaster itself. A
proper
matching
of the pore-size distribution of the plaster with that of the
substrate
is needed. Applying a plaster without knowledge of the substrate
might
even lead to more damage instead of less.
- An extensive description can be found in:
J. Petkovic, L. Pel, H.P. Huinink, K.Kopinga, and R.P.J.
van
Hees,
Salt transport and crystallization in plaster layers: a nuclear
magnetic
resonance study, 6th International Symposium on the Conservation
of
Monuments
in the Mediterranean Basin, 318-322, April 7-10, Lisbon,
Portugal
(2004).
J. Petkovic, L.Pel, H.P. Huinink, K.Kopinga, and R.P.J. van
Hees,
Salt
transport in plasters/substrate layers: a nuclear megnetic
resonance
study,
13th. Int. Brick and Block Masonry Conference, Amsterdam, July
4-7
(2004).
J. Petkovic, Moisture and ion transport in layerd porous
building
materials:
a Nuclear Magnetic Resonance study, Ph.D. thesis,
Eindhoven University of Technology, the Netherlands (2005).
J. Petkovic, H.P. Huinink, L. Pel, K. Kopinga and R.P.J. van
Hees,
Salt transport in plaster/substrate layers, Materials and Structures 40, 475-490 (2007)
 Back
to main page
Back
to main page



